Drug-eluting Stent
BRIEF DESCRIPTION
ANGIOPLASTY STENT, model: ALISCR L605 Rapamycin Eluting Coronary stent system.
PRODUCT CODE
ALISCR
COUNTRY OF ORIGIN
Germany
TRADE NAME
ALISCR L605 Rapamycin Eluting Coronary stent system.
APPROVAL BY THE NATIONAL ORGANIZATION FOR MEDICINES (NOM)
ISO 9001/ EN 13485 & CE
ANGIOPLASTY STENT, model: ALISCR L605 Rapamycin Eluting Coronary stent system (Cobalt Chromium enriched with Rapamycin), with biodegradable polymer, manufactured Germany. ISO 9001/ EN 13485 & CE certified.
ALISCR stent with Rapamycin is designed to be used in cardiology-procedures and is exclusively manufactured in-house.
It is a device/drug combination product comprised of two regulated components: a device (ALISCR L605 Coronary stent system) and a drug product (Rapamycin) contained in a biodegradable polymer coating. The innovation of ALISCR L605 stent is that the drug Rapamycin and its polymer are released biodegrading, six (6) weeks after implantation, so that a stripped ALISCR L605 stent remains. As a result, we have the best possible long-term effects for the patient. It is deliverable in different lengths and diameters (see below). The 2.5-4.0 mm diameter L605 Cobalt Chromium Alloy Stents use one design. The same stent is crimped on various size delivery catheter balloons. Because the identical stent component is used for the entire 2.5-4.0 mm diameter range, the total drug per stent is a function of its length, irrespective of its diameter.
ALISCR L605 Coronary stent system is a stent with a drug/polymer coating formulation consisting of Rapamycin (the active ingredient) and a polymer carrier. The drug/polymer coating is adhered to the entire surface (i.e., luminal and abluminal) of the stent.
The release rate of Rapamycin in ALISCR L605 stents is shown in the following graph:
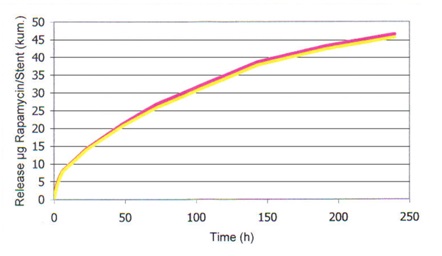
The stent is intended as a permanent implanted device, which presses atherosclerotic plaques against the vessel wall. It is expected to be overlaid with neointima cells and endothelium, until a cell layer is thoroughly established. Stents may be implanted singularly or in a multiple tandem, in order to maintain blood permeability of the vessel. They should be inserted only after the target lesion has been dilated using an appropriately sized balloon dilatation catheter. When unexpanded, the stent has an outer diameter of 0.94 mm. The Stent-System is packaged in sterile condition.
ALISCR L605 Coronary stent system with Rapamycin covers all modern technical requirements and in particular:
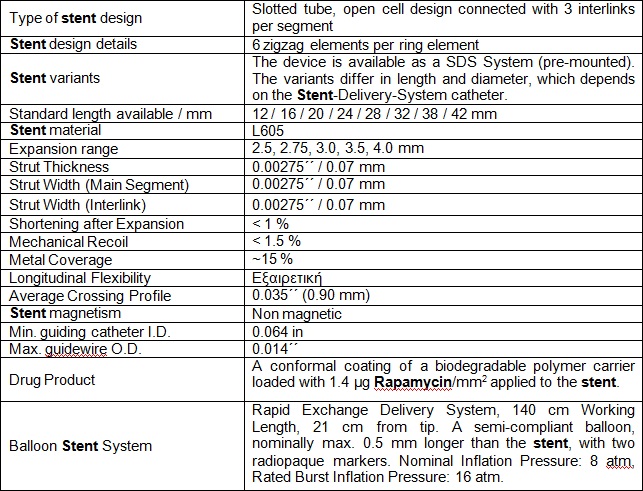
Balloon catheter
Stents should be inserted using an appropriately sized balloon dilatation catheter. This ALEX PTCA balloon catheter is intended for dilatation of stenoses in the coronary vessel system.
It is deliverable in different lengths and diameters. This is a double-lumen fast exchange catheter with a mounted balloon of a semi-compliant Nylon co-polymer material and a Hypo-Tube catheter shaft. At a nominal pressure of 8 bar = 0.8 Mpa the balloon reaches its nominal diameter. If the actual pressure remains under or exceeds the nominal pressure, the balloon diameter changes (see compliance table below).
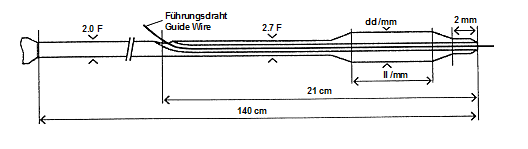
The first lumen starting distally (21 cm long) is provided for the introduction of a guidewire (0.014”), with its exit at the balloon sided end of the catheter. The second lumen with the Luer-connection serves as inflation and deflation lumen for the balloon. The usable catheter length is 140 cm. A proximal and a distal X-ray marker (platinum iridium ring) at the catheter balloon segment define the balloon length (cylindrical part) and allow correct positioning within the stenosis under X-ray control.
The structure of the catheter is shown in the following figure:
The balloon diameter, according to pressure put, is shown in the following compliance table (in vitro at 38 0C):
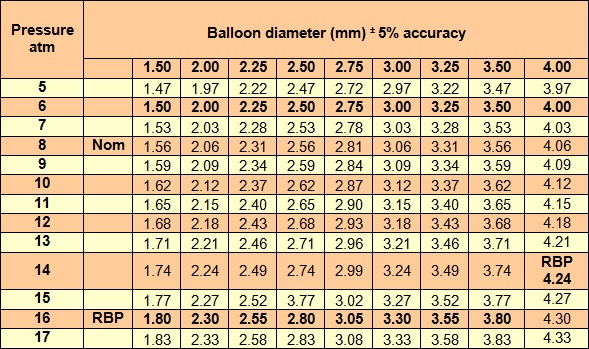
Nom: Nominal pressure, RBP:Rated Burst Pressure,Average Burst Pressure=22 atm
10% expansion from 8 atm to 16 atm,Average deflation
Device variants
The device is produced under the nameALISCR. The variations of the device regarding lengths and diameters (incl. Article numbers) are shown in the following tables.
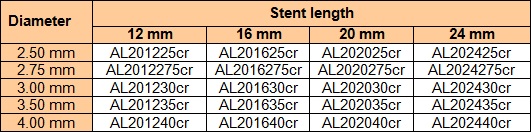
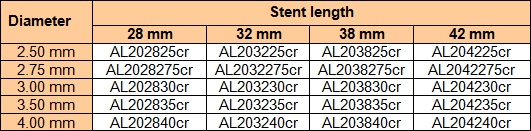
Amount of medicinal substance
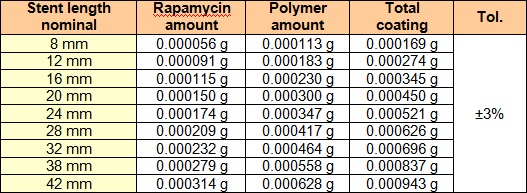
The medical device elutes Rapamycin after implantation. The amount of medicinal substance bounded with the Resomer-polymer onto the stent surface is shown in the table below (this corresponds to the nominal amount of Rapamycin of 1.4 μg / mm2 stent-surface).
Sterilization is achieved by ethylene oxide. This is a single-use product.
 English
English Greek
Greek
