Angiography Pack NL4
BRIEF DESCRIPTION
Angiography, coronary angiography, angioplasty pack
PRODUCT CODE
05-117639AA-03
COUNTRY OF ORIGIN
The Netherlands
TRADE NAME
PACK CARDIO NL4
APPROVAL BY THE NATIONAL ORGANIZATION FOR MEDICINES (NOM)
2820008863241
The PACK CARDIO NL4 is intended to cover the needs of the hemodynamic laboratory during coronary angiography and vascular procedures. It is a complete pack, made of high-quality materials that provide everything needed to prepare the surgical table. The manufacturing company, Medica Europe B.V., declares that all individual items are sourced from the USA, the UK, and European countries, and they are packaged in the Netherlands after undergoing strict quality control based on the MDR standard.
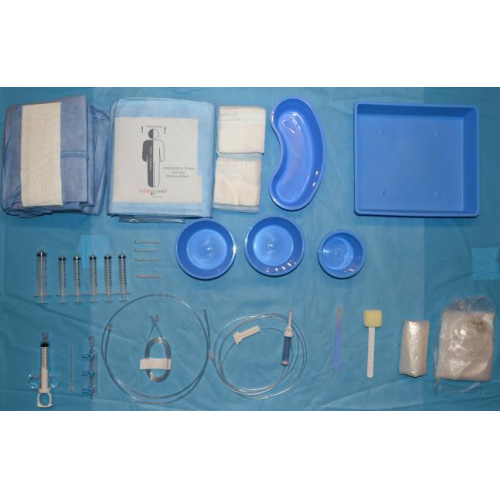
The pack includes the following materials:
|
S/N |
DESCRIPTION |
UNIT | |
|---|---|---|---|
|
1 |
Transparent protective cover 102Χ51cm, round |
1 |
|
|
2 |
Transparent protective cover 91Χ104cm, square |
1 |
|
|
3 |
Round Bowl 250ml, Blue |
1 |
|
|
4 |
Round Bowl 500ml, Blue |
2 |
|
|
5 |
Special sponge with plastic handle 19cm, for Betadine of foerster clamp type |
1 |
|
|
6 |
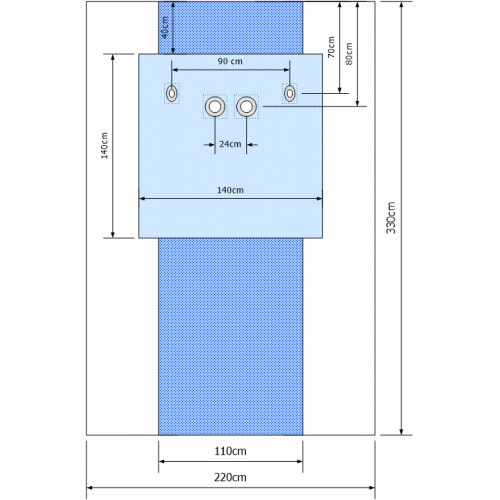
DRAPE FEM/RADIAL 3PLY radiology table drape with 4 openings for femoral and radial access and transparent along both sides 330X220cm, Blue |
1 |
|
|
7 |
10x10cm Gauze, 12ply |
10 |
|
|
8 |
Medical gown size: XL lined and hypoallergenic |
1 |
|
|
9 |
Hand towel 42.5X30.5cm |
1 |
|
|
10 |
PVC pressure connector 150cm, male/female |
1 |
|
|
11 |
3-way, right (multi-tap) manifold, with handles, off, (OB, 500 psi) |
1 |
|
|
12 |
Infusion device with drip chamber |
1 |
|
|
13 |
Syringe 2ml, luer slip |
1 |
|
|
14 |
Syringe 10ml, luer slip |
2 |
|
|
15 |
Σyringe 20ml, luer slip |
2 |
|
|
16 |
10ml coronary angiography syringe (thumb ring, finger rings) with stopper and rotating luer lock adapter (Socorex type) |
1 |
|
|
17 |
Surgical Table Cover 195Χ150cm, (waterproof, lined, absorbent), Blue |
1 |
|
|
18 |
Tray 28Χ25Χ5cm, Blue |
1 |
|
|
19 |
Needle 16GX4cm |
1 |
|
The Pack Cardio NL4 is available sterile and in disposable packaging, indicating Lot number and Reference for easier tracking of the material. It has ISO 13485:2016, EN ISO 13485:2016 & CE-MDR approval.
 English
English Greek
Greek